

So your application claims an unrealistic accuracy. The molar mass of sulphur depends on where you get it from The really good ones don't give the spurious accuracy that yours does. The commercial packages for calculating masses for chemical compounds let you choose which average you want. You can calculate as accurately as you like- but the real values are uncertain. This application does it in real-time while entering data. So compound doesn't have to be in database and right mass will appear.
Then when we split chemical compound to elements, look up database for masses of elements, and add them together, we have mass of compound. If we multiply isotope mass (from mass spectrometer) by natural abundance, repeat and add all other isotopes of same element we have average mass of element. It has database of the all isotopes (3142), database of the all elements, and database of thousands of chemical compounds. We do care about precise calculations.Īpplication visible on screen-shot is made by me.
#HOW TO CALCULATE PPM FROM MOLE FRACTION HOW TO#
We are simply disagreeing on interpretation how to proceed when there is just "ppm" alone. We are obviously agreeing on how to interpret: We're just differently interpreting lack of information in brackets after ppm. I am not wrong, and you're not just right. So disagreement between one method over other is 5.42 times (with 1000 ppm (per mass)).ġ000 ppm (per volume) is 0.1% (per volume) (with density p=1.84 g/cm 3) which is 1838.4557 ppm (per mass). So, your spreadsheet is either exactly right or roughly 2 million times more wrong than I was, or something else.ġ000 ppm (per molecules) is 0.542% (per mass) which is 5420 ppm (per mass). He needs to check with whoever asked him to make up the solution (unless he can be sure from the context that it doesn't matter (for example, if he's just washing glassware with it.).) Perhaps the most important point is that the person who asked the question at the start of the thread can't expect a valid answer. So, if I dissolve 1 mg of sugar in 1 ml of alcohol it's a 1000 ppm solution. I don't know what your first language is,but this page is in English.Īnd current English usage is decisively ambiguous.įor mixtures of gases ppm usually refers to mole fraction- but that's because it's (practically) the same as volume fraction.įor solutions it's as often a "shorthand" for mass of solid dissolved in a volume of solvent that would have 999999 times that mass, if the solvent were water.
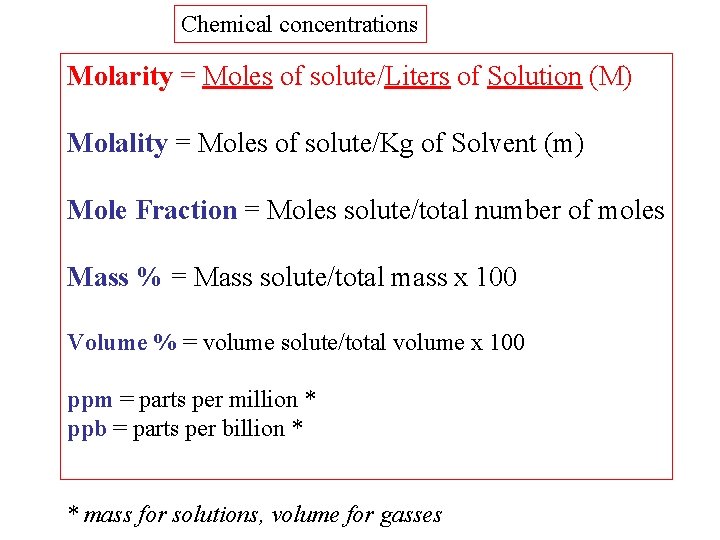
Well, OK, it's wrong- but it was early in the morning, and I had a bus to catch. "take 1 gram of acid and dilute it to a million grams with water" "take 1 gram of acid and dilute it with a million grams of water" I'm sure it's someone's "law" that, when you correct something on the internet you add mistakes of your own. When the abbreviation ppm does not have any additional information to the contrary, that we are talking about the mole fraction. " The unit ppm is also sometimes used as a way to express a fraction of the weight, but usually only in this case specifically inform the writing eg. This concentration is derived from the mole fraction and determines how much chemical compound per 1 million molecules of the solution." "ppm (parts per million) - way of expressing the concentration of a very dilute solutions of chemical compounds. On my language wikipedia page of ppm, there is said (google translator used): If you're calculating concentration by using m 1/m 2, you would not even pass exam in our high school. m 1/(m 1+m 2) - concentration of first substance, m 2/(m 1+m 2) - concentration of 2nd substance. You need to dilute with 999,999 to have right proportions. If you dilute 1 gram of substance with 1 million grams of water you won't have 1 ppm (by mass), but less 1/1,000,001. Unless you are dealing with gases the mass/volume interpretation is probably most common and you are wrong. Example \(\PageIndex\) summarizes the different units of concentration and typical applications for each.Or, do I take 1 gram of acid and dilute it with a million grams of water, or do I take a millilitre of acid and dilute it with a million millilitres of water?


 0 kommentar(er)
0 kommentar(er)
